(P 221) 8+ YEARS OF IMMUNOTHERAPY FOR METASTATIC ALVEOLAR SOFT PART SARCOMA
.jpg)
Benjamin C. Powers, MD (he/him/his)
Associate Professor
University of Kansas Cancer Center
Overland Park, Kansas, United States
Author(s)
Objective: To show long term response and survival in metastatic alveolar soft part sarcoma using immunotherapeutics
Methods: We present a young man diagnosed with Alveolar Soft Part Sarcoma from thigh in Aug 2012 with characteristic t(X;17). As is typical of this disease, his disease has been present in lung, liver, adrenal, bone, and brain. He had standard radiation and stereotactic radiation to variety of spots, Neuro-ablate to brain lesions, microwave ablation to liver, and laser treatments to lungs in Germany. Systemically, he had tried temozolomide on two different occasions and sunitinib.
By June 2016, CT c/a/p and bone scan showed interval progressive disease in chest, liver, adrenal and suprarenal fossa, with a stable MRI brain. He was down to 98 lbs, with little energy, little appetite or muscle mass to do anything. Realistic options included off-label use of single-agent nivolumab (compassionate use basis) vs home hospice. He was too sick to seek out immunotherapy trials. After approval, nivolumab was started late July 2016.
Results: He was started on nivolumab 240mg every 2-weeks. There was concern that the immunotherapy was causing intermittent "mania". He took a four month hiatus from Sept - Dec 2017 to see if that made things better. Despite this break in drug, he still had outbursts directed mostly towards his wife. Over time, this became more apparent this was stemming from marital issues, not immunotherapy-induced. He has had no major anger episodes since restarting on immunotherapy 1/3/18 and participating in marriage counseling.
By April 2019, significant growth was noted in a centrally located liver mass. He underwent bland embolization to this liver lesion.
Based on data from CTOS 2017 (1), we switched to combo of pembrolizumab 200mg IV q3wks + axitinib PO 5mg BID June 2019. CT October 2019, after 4 months of therapy, suggested interval enlargement of hepatic lesion, so this right hepatic lobe lesion was embolized. We stayed with same combination therapy. We dose-reduced axitinib to 2mg PO BID in hopes of better tolerance, then he stopped axitinib altogether in Feb 2020 after disliking the fatigue, mouth sores, and breathing issues. Single-agent pembrolizumab was continued then.
He had repeat cryoablation to this liver metastasis in Feb, May and Oct 2020. MRI abdomen March 2021 showed increase in size of enhancing tumor along the margin of ablation cavity with no other obvious active disease elsewhere. Therefore, we sent him for partial hepatectomy and cholecystectomy 4/23/21. Path was c/w refractory ASPS and chronic cholecystitis, respectively.
From late 2021 to present, there have been waxing and waning consolidative processes in anterior left upper lobe and posterior left lower lobe. We have biopsied these in Nov 2021, May 2022 and May 2023, all benign, suggesting non-tuberculous mycobacteria without indication for treatment given lack of symptoms.
Latest surveillance imaging was MRI head, CT chest and MRI abdomen in early June 2024 - no evidence of active disease.
Conclusion: Despite these resistant areas in the liver, he has been continued on pembrolizumab alone since Feb 2020. Immunotherapy has totally changed around his life physically since Summer 2016. He has gained 45 pounds back without getting a feeding tube! (Image 1) He has more energy, participates in yoga routinely, and is back to his master gardening around the house and community.
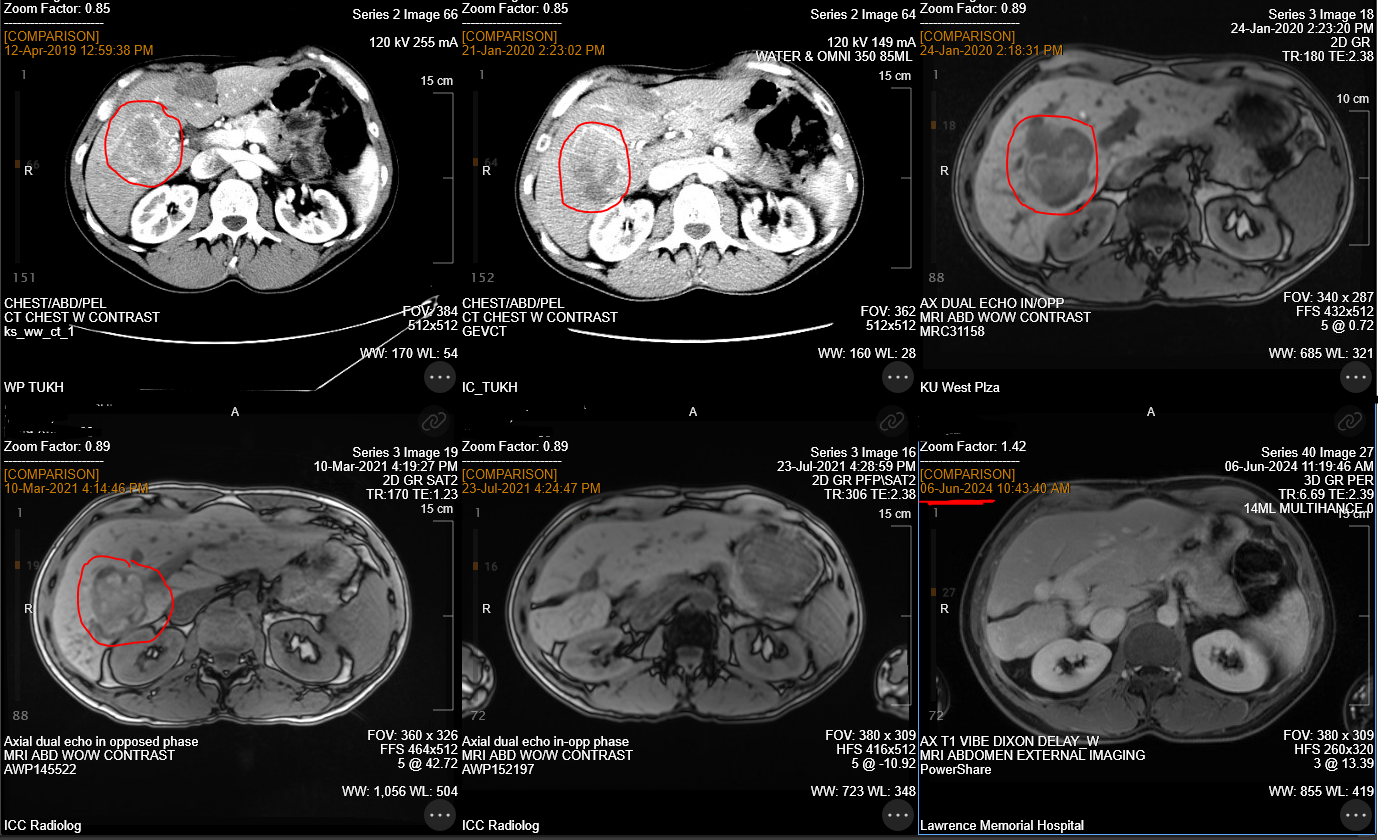
Ongoing use of immunotherapy should be entertained in alveolar soft part sarcoma, as it can produce long lasting response, improvement in quality of life. If areas of resistance pop up (image 2 - liver progression), then we would recommend aggressive local control measures while still continuing the immunotherapy.
(1) Wilky BA, Wieder E, Kolonias D, et al. Antitumor activity of axitinib plus pembrolizumab in a phase II trial for patients with advanced alveolar soft part sarcoma (ASPS) and other soft tissue sarcoma. Oral presentation at: Connective Tissue Oncology Society (CTOS) 2017 Annual Meeting. November 8-11, 2017; Maui, Hawaii.
Methods: We present a young man diagnosed with Alveolar Soft Part Sarcoma from thigh in Aug 2012 with characteristic t(X;17). As is typical of this disease, his disease has been present in lung, liver, adrenal, bone, and brain. He had standard radiation and stereotactic radiation to variety of spots, Neuro-ablate to brain lesions, microwave ablation to liver, and laser treatments to lungs in Germany. Systemically, he had tried temozolomide on two different occasions and sunitinib.
By June 2016, CT c/a/p and bone scan showed interval progressive disease in chest, liver, adrenal and suprarenal fossa, with a stable MRI brain. He was down to 98 lbs, with little energy, little appetite or muscle mass to do anything. Realistic options included off-label use of single-agent nivolumab (compassionate use basis) vs home hospice. He was too sick to seek out immunotherapy trials. After approval, nivolumab was started late July 2016.
Results: He was started on nivolumab 240mg every 2-weeks. There was concern that the immunotherapy was causing intermittent "mania". He took a four month hiatus from Sept - Dec 2017 to see if that made things better. Despite this break in drug, he still had outbursts directed mostly towards his wife. Over time, this became more apparent this was stemming from marital issues, not immunotherapy-induced. He has had no major anger episodes since restarting on immunotherapy 1/3/18 and participating in marriage counseling.
By April 2019, significant growth was noted in a centrally located liver mass. He underwent bland embolization to this liver lesion.
Based on data from CTOS 2017 (1), we switched to combo of pembrolizumab 200mg IV q3wks + axitinib PO 5mg BID June 2019. CT October 2019, after 4 months of therapy, suggested interval enlargement of hepatic lesion, so this right hepatic lobe lesion was embolized. We stayed with same combination therapy. We dose-reduced axitinib to 2mg PO BID in hopes of better tolerance, then he stopped axitinib altogether in Feb 2020 after disliking the fatigue, mouth sores, and breathing issues. Single-agent pembrolizumab was continued then.
He had repeat cryoablation to this liver metastasis in Feb, May and Oct 2020. MRI abdomen March 2021 showed increase in size of enhancing tumor along the margin of ablation cavity with no other obvious active disease elsewhere. Therefore, we sent him for partial hepatectomy and cholecystectomy 4/23/21. Path was c/w refractory ASPS and chronic cholecystitis, respectively.
From late 2021 to present, there have been waxing and waning consolidative processes in anterior left upper lobe and posterior left lower lobe. We have biopsied these in Nov 2021, May 2022 and May 2023, all benign, suggesting non-tuberculous mycobacteria without indication for treatment given lack of symptoms.
Latest surveillance imaging was MRI head, CT chest and MRI abdomen in early June 2024 - no evidence of active disease.
Conclusion: Despite these resistant areas in the liver, he has been continued on pembrolizumab alone since Feb 2020. Immunotherapy has totally changed around his life physically since Summer 2016. He has gained 45 pounds back without getting a feeding tube! (Image 1) He has more energy, participates in yoga routinely, and is back to his master gardening around the house and community.
Ongoing use of immunotherapy should be entertained in alveolar soft part sarcoma, as it can produce long lasting response, improvement in quality of life. If areas of resistance pop up (image 2 - liver progression), then we would recommend aggressive local control measures while still continuing the immunotherapy.
(1) Wilky BA, Wieder E, Kolonias D, et al. Antitumor activity of axitinib plus pembrolizumab in a phase II trial for patients with advanced alveolar soft part sarcoma (ASPS) and other soft tissue sarcoma. Oral presentation at: Connective Tissue Oncology Society (CTOS) 2017 Annual Meeting. November 8-11, 2017; Maui, Hawaii.

