(P 68) FEASIBILITY OF FIVE-DRUG INTERVAL-COMPRESSED CHEMOTHERAPY FOR ADOLESCENTS AND YOUNG ADULTS WITH EWING SARCOMA FAMILY TUMORS IN THE REAL-WORLD SETTING IN OMAN

Boris Itkin, MD, MSc
Sr. Consultant Medical Oncologist
SQCCCRC
Seeb, Masqat, Oman- MH
Muhammad Rashid Hanif, MD
MD
SQCCCRC
Muscat, Masqat, Oman - DA
Doaa Abbas, MD, MSc
MD
SQCCCRC
Muscat, Masqat, Oman - SU
Saif Ur Rab, MD
SQCCCRC
Muscat, Masqat, Oman
Author(s)
Co-Author(s)
Co-Author(s)
Objective: Data on the feasibility of interval-compressed Vincristine Doxorubicin Cyclophosphamide Ifosfamide Etoposide (VDC/IE) outside of leading sarcoma centers are scarce. We studied actual dose density and delays in the administration of interval-compressed VDC/IE in the first six cycles of treatment in adolescent and young adult (AYA) patients with Ewing Sarcoma Family Tumors in a single institution in Oman aiming to estimate:
-median duration of the cycle,
-percentual exceedance of the overall treatment length relative to its ideal duration (PEOTD) and
-explore causes of delays.
Methods: Previously untreated patients with Ewing Sarcoma Family Tumors of any stage aged between 12 and 40 years old who underwent interval-compressed VDC/IE chemotherapy at Sultan Qaboos Comprehensive Cancer Care and Research Center, Oman from 01.08.2021 to 20.05.2024. were eligible for this study. All patients were treated outside of clinical trials. Supportive treatment, dose adjustments, and treatment delays were decided by the treating physician on an individual basis following the institutional guidelines. We retrospectively extracted from electronic clinical records clinicodemographic data and dates of chemotherapy cycles to start computing overall and per-patient cycle duration. we computed the cycle duration as the time interval between Day 1 of two contiguous cycles. We calculated the Percentual Exceedance of the overall treatment length relative to its ideal duration (PEOTD) by dividing the actual by the ideal treatment duration. The ANOVA test was used for between-cycle length comparison at 0.05 alpha level.
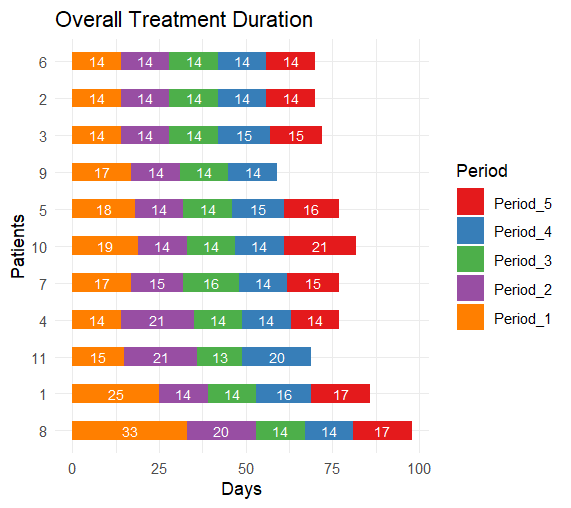
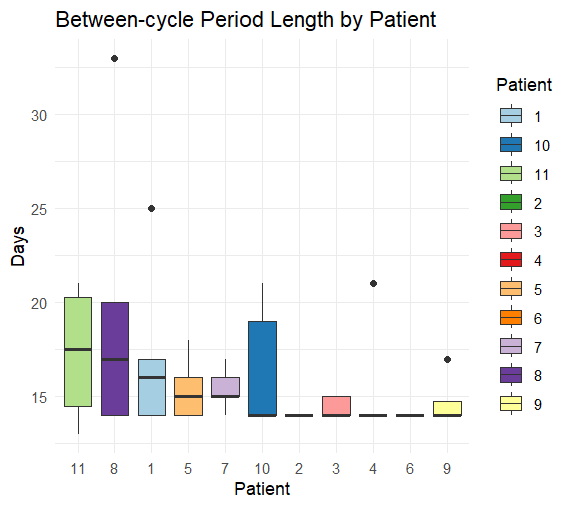
Results: Of the 13 potentially eligible patients 11 met eligibility criteria. The median age was 16 years, interquartile range (IQR) 14-20 years. The histopathological and molecular diagnoses were Ewing Sarcoma in 10/11 patients and BCOR-rearranged sarcoma in 1/11 patients. Eight patients had localized and three metastatic stages. 9/11 patients completed six cycles and 2/11 completed five cycles of interval compressed VDC/IE chemotherapy resulting in five between-cycle intervals in 9 patients and four between-cycle intervals patients in 2 patients respectively. A total of 53 between-cycle intervals were analyzed. The median cycle duration was 14 days, IQR 14-16 days. The median per-patient cycle duration varied from 14 to 17.5 days, median = 14 days. The median overall treatment duration was 77 days, IQR 70-79.5 days. The median PEOTD was 10%, IQR 4.1% – 20%. A trend to a longer cycle duration in the metastatic versus localized stage was not statistically significant, p = 0.0625. Delays were caused by chemotherapy toxicity in 67%, bed unavailability in 22%, and patient non-compliance in 11%.
Conclusion: Interval-compressed VDC/IE chemotherapy is feasible in real-world AYA patients with Ewing Sarcoma Family Tumors in Oman. The overall dose density was satisfactory. Although chemotherapy toxicity was the main cause of the delays, bed unavailability and patient non-compliance compliance were significant, potentially modifiable contributors to the delays.
.png)
-median duration of the cycle,
-percentual exceedance of the overall treatment length relative to its ideal duration (PEOTD) and
-explore causes of delays.
Methods: Previously untreated patients with Ewing Sarcoma Family Tumors of any stage aged between 12 and 40 years old who underwent interval-compressed VDC/IE chemotherapy at Sultan Qaboos Comprehensive Cancer Care and Research Center, Oman from 01.08.2021 to 20.05.2024. were eligible for this study. All patients were treated outside of clinical trials. Supportive treatment, dose adjustments, and treatment delays were decided by the treating physician on an individual basis following the institutional guidelines. We retrospectively extracted from electronic clinical records clinicodemographic data and dates of chemotherapy cycles to start computing overall and per-patient cycle duration. we computed the cycle duration as the time interval between Day 1 of two contiguous cycles. We calculated the Percentual Exceedance of the overall treatment length relative to its ideal duration (PEOTD) by dividing the actual by the ideal treatment duration. The ANOVA test was used for between-cycle length comparison at 0.05 alpha level.
Results: Of the 13 potentially eligible patients 11 met eligibility criteria. The median age was 16 years, interquartile range (IQR) 14-20 years. The histopathological and molecular diagnoses were Ewing Sarcoma in 10/11 patients and BCOR-rearranged sarcoma in 1/11 patients. Eight patients had localized and three metastatic stages. 9/11 patients completed six cycles and 2/11 completed five cycles of interval compressed VDC/IE chemotherapy resulting in five between-cycle intervals in 9 patients and four between-cycle intervals patients in 2 patients respectively. A total of 53 between-cycle intervals were analyzed. The median cycle duration was 14 days, IQR 14-16 days. The median per-patient cycle duration varied from 14 to 17.5 days, median = 14 days. The median overall treatment duration was 77 days, IQR 70-79.5 days. The median PEOTD was 10%, IQR 4.1% – 20%. A trend to a longer cycle duration in the metastatic versus localized stage was not statistically significant, p = 0.0625. Delays were caused by chemotherapy toxicity in 67%, bed unavailability in 22%, and patient non-compliance in 11%.
Conclusion: Interval-compressed VDC/IE chemotherapy is feasible in real-world AYA patients with Ewing Sarcoma Family Tumors in Oman. The overall dose density was satisfactory. Although chemotherapy toxicity was the main cause of the delays, bed unavailability and patient non-compliance compliance were significant, potentially modifiable contributors to the delays.

.png)

